GUIDE TO THE EXPOSOME
Why Exposome Studies are Needed
2.0 Introduction
Having defined the components of the exposome and touched on tools used to perform exposome research in Chapter 1, we will discuss various study designs for exposome research in this chapter. Exposome research represents a transformative paradigm in environmental health science that systematically captures the cumulative and dynamic interplay between environmental exposures and human biology across the lifespan1. This comprehensive approach recognizes that health outcomes do not arise from isolated exposures, but rather, from complex temporal (i.e., progressive) patterns of environmental factors that accumulate and exert differential effects depending on critical developmental windows of susceptibility. This chapter will focus on two elements not typically captured by current research structures: 1) the impact of exposure time on health across one’s lifetime and 2) the cause-and-effect relationship between exposures and disease onset. This chapter leverages key concepts that have been previously introduced under the exposome framework, including ‘meet in the middle’ and ‘biography-to-biology’, which aim to connect early life exposure(s) to long-term health outcomes, and ‘triangulation’, which utilizes multiple assumptions to determine relationships that are causal rather than simply correlative2,3. Metabolomics is vital to addressing the knowledge gaps related to these concepts because it most closely reflects the impact of both recent and cumulative exposures on the phenotype. It is also well-suited for longitudinal studies that profile samples collected over time and evaluate those results alongside detailed exposure data; an approach that is necessary to aligning exposure timing with biological responses4–6.2.1 Temporal Exposome
2.1.1 From Biography to Biology: The Temporal Journey of Exposure across a Lifetime
The life-course perspective is essential to exposomics, recognizing that exposures during vulnerable developmental periods, especially the prenatal environment, early childhood, and adolescence, can cause lasting biological changes that may not manifest as disease until many years later. Evidence increasingly shows that pre- and early post-natal exposure to endocrine-disrupting chemicals, such as per- and polyfluoroalkyl substances (PFAS) and bisphenol A (BPA), have detrimental effects on growth and development7-9. These effects are linked to altered metabolic programming that raises the risk for obesity, cardiometabolic problems, and neurodivergent behaviors later in childhood9,10.
Environmental insults during critical windows can negatively affect health through various mechanisms, including epigenetic changes, alterations in organ development, and disruptions to hormonal signaling pathways11. Capturing this intricate transition from exposure to biological outcome necessitates prospective, longitudinal cohort studies with repeated collection of diverse biospecimens and comprehensive exposure assessments over time. This discovery-based approach is necessary to establish the timing of environmental influences and their subsequent effects on human health. Viewing these studies through the exposome framework thus elevates our causal understanding of disease onset from simple static risk factors to a dynamic continuum where lifelong environmental experiences gradually shape biological systems and influence health outcomes across generations1,12.
A powerful way to bridge the gap between early exposure and long-term disease risk is the ‘meet in the middle’ (MITM) concept proposed by Vineis et al2.
This strategy aims to connect early biomarkers of response to the long-term development of chronic disease. It involves:
-
- Measuring intermediate biomarkers through agnostic -omic investigations.
- Relating these biomarkers retrospectively to measurements of external exposure.
- Relating them prospectively to the ultimate health outcome.
For example, the EXPOsOMICs project13 applied the MITM approach to investigate cerebro-cardiovascular disease (CCVD) risk14,15. The study used bio-banked blood samples to perform detailed analyses of relevant biomarkers, including inflammatory proteins, genome-wide DNA methylation, and metabolites. The findings revealed:
-
- An enrichment of altered DNA methylation in genes related to reactive oxygen species/glutathione/cytotoxic granules and cytokine signaling pathways. These alterations were significantly associated with both air pollution exposure and CCVD risk.
- Interleukin-17 levels were associated with higher exposure to NO2 and NOx, and also with an increased risk of CCVD15.
- Metabolomic investigations revealed disruptions in the linoleate metabolism pathway, which is crucial for immune and inflammatory responses14. This disruption was linked to both air pollution exposure and a higher risk of CCVD and asthma.
These results show that epigenetic and metabolomic signals can serve as intermediates between environmental exposures and disease outcomes, offering clear examples of pathways to disease that deserve further study. In this context, environmental, lifestyle, and psychosocial factors cause heritable and lasting epigenetic changes (including DNA methylation), which build up as “epigenetic drift” and influence long-term health and metabolism.
Beyond the concept of meet in the middle, the exposome framework can also provide insight into exposures that impact the rate of biological aging, which has implications for early development of diseases typically seen in older populations, including cancer, heart disease, and hypertension. For example, Nassan et al16 employed Metabolon’s Global Discovery Panel to analyze participants in the Normative Aging Study to characterize relationships between long-term exposure to the air pollutants PM2.5, O3, and NO2, and changes to metabolites that are also markers of biological aging16. They found that long-term exposure, specifically to PM2.5, was associated with metabolic perturbations in nucleic acids, various amino acids, and lipids. The affected pathways are linked to inflammation, oxidative stress, immunity, and nucleic acid damage and repair, all biological processes underlying age-related pathologies leading to arthritis, diabetes, and cancer.
This concept of exposures contributing to one’s biological age was demonstrated by another study, which aimed to develop an age-predictive metabolite biomarker panel17. Here, ~12,000 adult metabolomes were profiled, and the large metabolomic age model was restricted to features that Metabolon’s Global Discovery Platform could consistently and reliably measure. The final age- predictive panel prominently featured lipids and xenobiotics. The lipid pathways identified in this study were the same ones shown to shift with air pollution in the study cited in the above paragraph, showing that pollution-driven changes can register as older metabolomic age.
Overall, these studies show the importance of viewing exposure-to-disease hypotheses through the lens of the exposome framework. In the next section, we discuss another aspect of this framework, which addresses the idea of ‘finite’ versus ‘continuous’ outcomes.
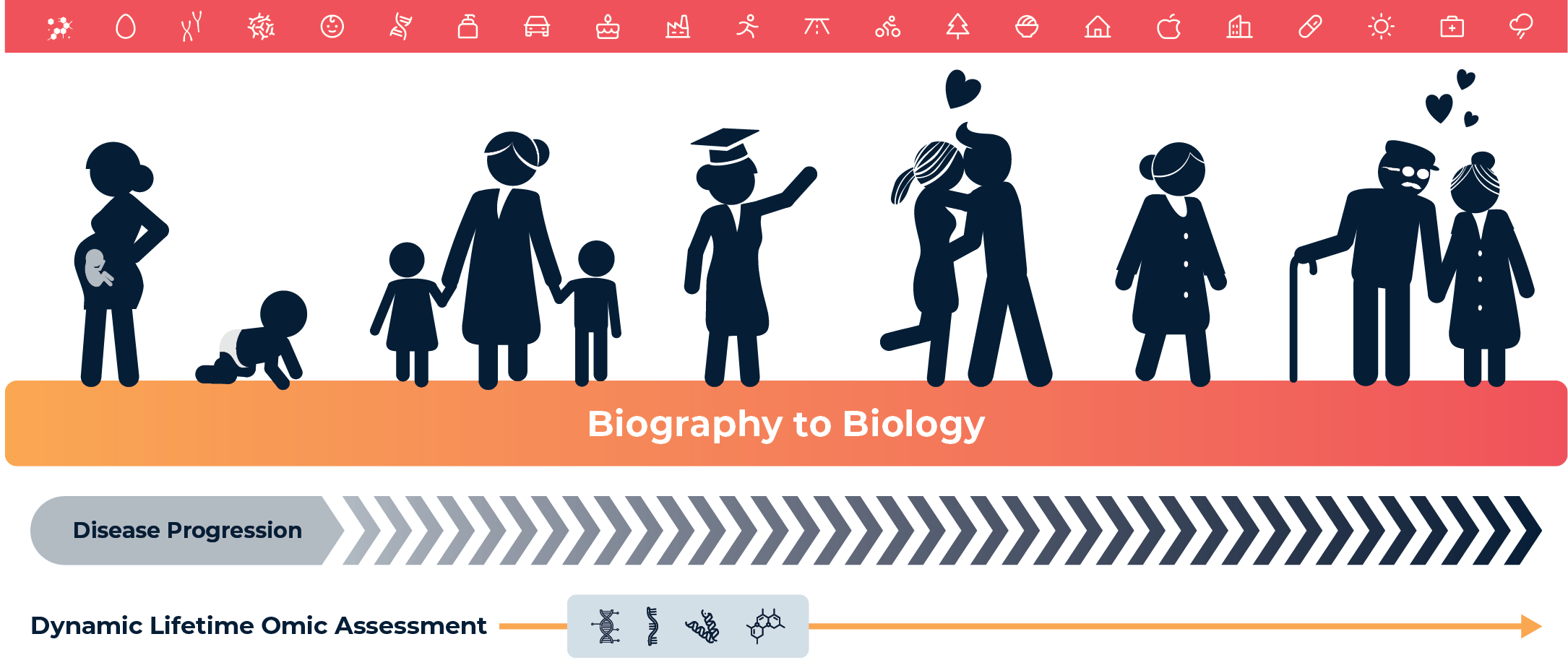
Figure 2.1: The “Biography to Biology” exposome concept illustrates how an individual’s lived experience encompassing lifestyle, diet, environment, behavior, geography, and social determinants, translates into measurable biological outcomes through molecular signatures captured in the metabolome. This conceptual model bridges personal history (“biography”) with systems-level biological expression (“biology”), highlighting the exposome as the dynamic interface through which cumulative exposures during critical windows shape health trajectories, disease risk, and therapeutic response over time.
2.1.2 The Temporal Journey of Disease, Beyond ‘Rare’ or ‘Hard’ Outcomes’
For decades, epidemiological research on the health effects of environmental stressors has been guided by a focus on clear, definitive, and severe health events. These “hard outcomes” such as mortality, a formal cancer diagnosis, disease exacerbations leading to hospitalization, or a heart attack, among others, have served as the primary endpoints for assessing risk, which typically manifest later in life2. However, long before a clinical diagnosis is made, environmental exposures can trigger a cascade of subtle, sub-clinical perturbations throughout the body. These early, often asymptomatic, changes represent the first footprints of an exposure on our biology and are the most sensitive indicators of environmental stress. The long latency periods of many noncommunicable diseases make traditional epidemiological studies less effective for identifying targets for early preventive measures.
The ability to capture these effects over time is a direct extension of the “biography-to-biology” approach, allowing researchers to see the gradual inscription of environmental influences onto an individual’s health. Omic technologies are instrumental in identifying these early molecular changes, which serve as novel response biomarkers. Their detection allows for earlier population surveillance and more precise risk assessment. Focusing on these sub-clinical endpoints is the key to unlocking a more proactive and preventive approach to medicine. Hard outcomes are late-stage events and by the time they occur, the window for simple, effective intervention may have closed. Sub-clinical markers provide an opportunity to identify at-risk individuals and populations when biological processes are just beginning to go awry, allowing for interventions, whether behavioral, environmental, or therapeutic, to be deployed when they are most likely to succeed.
The exposome paradigm, which emphasizes capturing the earliest biological responses to exposure, is inherently designed to reveal these critical pre-disease states. This was demonstrated by a study that hypothesized individuals exposed to perfluoroalkyl substances (PFOS and PFOA) in the womb and/or early childhood may face a higher risk of certain childhood diseases7. The researchers used Metabolon’s Global Discovery Panel to analyze the plasma metabolomes of a Danish cohort of 738 pregnant women and their children. Advanced statistical analyses identified causal links between exposure to PFOS and PFOA and childhood infections, asthma, and atopic dermatitis. High maternal exposure to these chemicals showed a significant association with non-atopic asthma by age 6, primarily driven by prenatal exposure rather than exposure during childhood. Overall, this study uncovered metabolic changes that may signal exposure-related preclinical metabolic shifts likely to lead to disease later on. This hypothesis- generating research could lay the groundwork for preventive strategies, especially for childhood asthma and demonstrates the potential of evaluating preclinical changes triggered by exposures.
2.2 Establishing Causality through Exposome Framework
2.2.1 Where Causality is Uncertain
Establishing a definitive causal link between an environmental exposure and a human disease is one of the most formidable challenges in modern science. The path from exposure to outcome is often long and obscured by many things, including a fog of confounding variables, the potential for reverse causation (i.e., the disease influences exposure-related behaviors), and the inherent limitations of observational study designs. The International Agency for Research on Cancer (IARC) ‘s classification of outdoor air pollution as a Group 1 carcinogen (“carcinogenic to humans”) stands as a landmark achievement in causal inference, a statistical method used to determine whether relationships between variables are truly cause-and-effect rather than simply correlative18. This conclusion was reached after a meticulous, multi-year review of a vast body of evidence, including epidemiological studies in humans, cancer bioassays in animals, and extensive data on the mechanistic pathways through which pollutants cause DNA damage, oxidative stress, and inflammation18.
The evidence for most other environmental exposures, however, is far less complete. This leads to significant scientific debate and, critically, divergent regulatory actions worldwide. The case of the herbicides, paraquat and diquat provide a stark illustration of how differing standards of evidence can create vastly different public health policies. This regulatory split is not born from different data sets, but from different interpretations of the same, often uncertain, evidence. The European Food Safety Authority (EFSA) identified “a critical area of concern” for paraquat and diquat, with models showing that worker exposure could exceed acceptable safety levels by several thousand percent, justifying action under a precautionary principle19. In contrast, the U.S. Environmental Protection Agency (EPA), while acknowledging the acute toxicity of paraquat, has concluded that the body of epidemiological evidence linking it to Parkinson’s disease is inconsistent and fraught with studies of varying quality, and therefore does not meet its threshold to establish a clear causal relationship20.
2.2.2 Triangulation: Strengthening evidence between exposure and disease
The regulatory divergence concerning paraquat and diquat presents a classic scenario where the exposome framework, particularly through the lens of triangulation, could provide the necessary scientific strategy to overcome this regulatory disparity. Vineis et al.221 proposed the triangulation concept as the ‘strategic use of multiple approaches to address one question,’ each relying on distinct, unrelated assumptions to strengthen causal inference.
In the following section, we present a hypothetical case study applying the exposome framework proposed by Sarigiannis et al3 to the herbicide diquat, illustrating how such an approach can generate the evidence base needed to inform policy and regulatory decision-making. This framework is organized across four interconnected pillars, integrating data from external exposures, internal exposures, and the temporal dimension of the exposome. By uniting these domains, the framework moves beyond the boundaries of classical epidemiology to incorporate high-resolution molecular phenotyping, multi-omics integration, and systems-level analyses.
2.2.3 First pillar: Comprehensive external exposure integration
The exposome paradigm assesses multi-factorial external exposures as a whole and thus would move beyond isolated studies assessing diquat as a single agent to viewing it as part of the complex mixtures that individuals are exposed to due to agriculture’s increasing reliance on pesticides to improve crop yields. This framework can evaluate multiple chemical and non-chemical stressors together, mapping out an “expotype” that reflects real-life complexity3. Methodologies include: 1) developing high-resolution geospatial models to estimate environmental concentrations in air, water, and soil, 2) personal exposure monitoring to measure direct contact among occupational and residential groups, and 3) dietary intake assessment by analyzing food residues22. These combined methodologies establish a comprehensive profile of external diquat exposure. This first pillar establishes the foundational evidence layer: the external stressor’s nature and magnitude.
2.2.4 Second pillar: Internal dosimetry and biomonitoring
The second layer connects external exposure and how much of it is actually absorbed into the body and aims to understand its retention and timing. This can be facilitated by traditional non-invasive collection methods, such as blood and urine, or known bioaccumulation tissue sites, such as liver or kidney biopsies. Physiologically based biokinetic modeling (PBBK) can be employed to stimulate the adsorption, distribution, metabolism, and excretion of diquat in the human body. PBBK is a bidirectional link between aggregate external exposure and internal dose. Current research on dosing on diquat relies heavily on high-dose rodent studies, so direct human biomonitoring is essential to reflect real-world, often lower-level, chronic exposures. For diquat, targeted biomonitoring of parent compound and metabolite by-products anchors exposure at the level of blood, urine, and tissues most at risk including the gut, liver, and kidney. Combining measured concentrations with PBBK-predicted tissue kinetics yields comparable dose metrics across populations and life stages.
2.2.5 Third pillar: Mechanistic insights through omics
The most powerful triangulation occurs when external exposure and internal dose findings align with molecular evidence of effect. To establish biological plausibility, omics technologies should be used to identify the molecular perturbations resulting from diquat exposure. Each omics layer can identify early biological alterations that connect internal dose with clinical and sub-clinical endpoints, the core of the ‘temporal exposome’ from the previous section.
Current evidence indicates that diquat’s primary toxic mechanism involves the generation of reactive oxygen species, leading to oxidative stress and subsequent cellular damage23. The kidney is the primary target organ of toxicity; however, lung and nervous system damage, along with gut dysbiosis leading to multi-organ failure, have been reported.
An advanced mechanistic approach would build upon this by:
-
- Utilizing Advanced In Vitro Models: Employing human-derived intestinal organoids or “gut-on-a-chip” systems to model the human intestinal epithelium. These models would be exposed to physiologically relevant concentrations of diquat to precisely quantify impacts on:
- Barrier Integrity: Measuring changes in transepithelial electrical resistance (TEER) and the expression of tight junction proteins like ZO-1 and occludin.
- Mitochondrial Function: Assessing mitochondrial membrane potential, ATP production, and the induction of mitophagy, as mitochondrial dysfunction is a known consequence of diquat exposure24.
- Deploying Multiomics Profiling: Agnostically interrogating the molecular sequelae of diquat exposure in these advanced models.
- Metabolomics: underpins the mechanistic strategy to reveal the functional consequences of disruptions in the other omic layers. For example, metabolomics directly reveals the functional consequences of diquat’s impact on the gut microbiota and host metabolism, by showing significant decrease in crucial microbial-derived metabolites like indole-3-methanol, 5-hydroxyindole-3-acetic acid, butyrate, and uridine, which are vital for maintaining intestinal barrier integrity and immune tolerance25. Thus, further studies would explore the relation between microbial shifts, such as reduced Lactobacillus and increased pathogenic bacteria like Ruminococcace, with these metabolite changes. Metabolomics profiles can also point to dysregulation of metabolic processes that are directly governed by transcript and protein activity. The observed decrease in microbial-derived metabolites correlates with increased inflammation and intestinal barrier dysfunction, which is directly linked to the downregulation of tight junction proteins (e.g., ZO-1, occludin, claudin-1) and the activation of inflammatory proteins and pathways such as NF- κB and MAPK pathway components. Diquat promotes ROS production, activating the NF-κB signaling pathway and enhancing phosphorylation of key proteins in the MAPK pathway (p38, ERK1/2, JNK)23. This activation leads to a massive release of pro-inflammatory cytokines (e.g., TNF-α, IL-6, IL-1β), triggering inflammation and further suppressing the expression of tight junction proteins, creating a vicious cycle of inflammation and barrier damage23. Together with metabolomics, the underlying molecular mechanisms that inhibit nutrient absorption and disrupt energy metabolism can be better understood.
- Transcriptomics and Proteomics: Analyze gene and protein expression profiles in accessible tissues (e.g., blood, tissues and cells) To identify molecular initiating events (ROS generation) that lead to adverse cellular outcomes (barrier dysfunction and apoptosis). This can reveal the activation of key signaling pathways, including those governing inflammation (e.g., NF-κB, MAPK) and antioxidant response pathways (e.g., Nrf2), providing mechanistic evidence linking exposure to cellular damage.
- Metagenomics: Recent studies have identified the gut as a key target of diquat toxicity23,26. For example, diquat has been shown to alter gut microbiota composition by reducing the abundance of Lactobacillus while enriching pathogenic bacteria, Actinobacteria and Ruminococcaceae, thereby exacerbating both intestinal and systemic toxicity25. However, the exact molecular mechanisms linking gut-specific injury to systemic organ failure remain unclear and need to be replicated in human studies. Furthermore, evidence showing how gut microbiota-derived metabolites modulate diquat toxicity is scarce. Lastly, it remains to be seen if targeting intestinal integrity or microbial balance alleviates multiple organ dysfunction. Applying 16S rRNA sequencing and shotgun metagenomics provides taxonomic information and offers deeper insights into the functional potential of the gut microbiome.
- Utilizing Advanced In Vitro Models: Employing human-derived intestinal organoids or “gut-on-a-chip” systems to model the human intestinal epithelium. These models would be exposed to physiologically relevant concentrations of diquat to precisely quantify impacts on:
2.2.6 Fourth pillar: Contextual insights and sociodemographic factors through observation
In the exposome paradigm, it is recognized that chemical exposure does not occur in a vacuum and that the influence of social and demographic conditions is not a confounder but a covariate that defines the exposome, shaping susceptibility and exposure patterns across windows of vulnerability. This general external exposome can be explored through observational studies that collect data on diet, lifestyle, occupational exposure, and socioeconomic status. Studies exploring these factors are needed to determine how individually based variables modify susceptibility to harmful levels of exposure, and to pinpoint vulnerable subpopulations. For example, the exposure risk of agricultural workers or residents living in areas with high diquat use is also influenced by factors like socioeconomic status and diet, which must be considered to gain a comprehensive understanding of population vulnerability.
2.2 Bridging the Regulatory Divide
This multi-tiered exposome framework operationalizes triangulation to generate streams of evidence that mutually reinforce each other and collectively strengthen causal inference. By integrating epidemiological observations, internal dose measurements, and mechanistic molecular insights, the approach establishes a robust chain of evidence linking exposure to health outcomes. This framework and use of triangulation have been successfully applied to other exposome paradigms, such as the HERACLES cohort27. It is important to note that other analytical approaches, such as Mendelian randomization (MR), can be applied to exposome research. However, MR relies on strict assumptions to establish causal inference, and in cases involving exposures like air pollution or pesticides, these assumptions are often violated, limiting its applicability.
In the case of diquat, regulatory divergence underscores the importance of such an approach: the European Union’s ban is grounded in precautionary toxicology and hazard identification, whereas the U.S. EPA highlights a lack of definitive epidemiological causality. An exposome-guided triangulation framework addresses this evidentiary gap by leveraging complementary methodologies, with metabolomics serving as the central anchor:
-
- Multiomics integration: Metabolomics provides the unifying foundation by uniquely capturing both exogenous metabolites that encapsulate the external exposure and linking this with endogenous perturbations. This mechanistically links with transcriptomics, proteomics, and metagenomics, enabling a comprehensive systems-level perspective and establishing strong biological plausibility.
- Computational models: extend this molecular evidence to predictive risk assessments, simulating diverse exposure scenarios and potential health trajectories.
- Exposure biomarkers: deliver quantifiable, human-relevant measures of internal dose, translating environmental presence into biological impact.
- Cross-cohort comparisons: validate findings at the population level, reinforcing generalizability and ensuring regulatory confidence.
2.4 Summary
The exposome offers a transformative way to understand health by capturing the full range of environmental, lifestyle, and social factors that shape biology across the human lifespan. Rather than viewing disease as the product of single exposures or isolated risk factors, the exposome allows us to track how these complex and cumulative influences are inscribed onto our biology over time. With advanced technologies such as metabolomics and multiomics, researchers can now detect the earliest molecular changes triggered by these exposures, often decades before clinical disease appears.
Environmental factors impact nearly every biological system. Pesticides like diquat illustrate how external chemicals can disrupt gut integrity, immune regulation, and systemic metabolism. Similarly, air pollution accelerates biological aging and drives inflammatory and cardiovascular pathways. Endocrine-disrupting compounds such as BPA or PFAS alter hormonal signaling and metabolic programming during critical windows of development, while nutritional and lifestyle factors shape energy metabolism, mitochondrial function, and even brain health. Across these diverse examples, the exposome framework connects seemingly unrelated external forces to common biological networks of stress, repair, and adaptation.
By integrating molecular, demographic, and environmental data, exposome-based research enables us to disentangle these complex interactions. This approach provides stronger causal evidence to guide preventive strategies, improve regulatory decisions, and identify novel targets for intervention. Ultimately, the exposome reframes health from a reactive model focused on treating late- stage disease to a proactive model centered on early detection, prevention, and resilience. As the ability to measure and interpret the exposome grows, it will transform how we understand the origins of disease and empower a new era of precision public health.
References
1. Wild, C. P. The exposome: from concept to utility. Int. J. Epidemiol. 41, 24–32 (2012).
2. Vineis, P. et al. What is new in the exposome? Environ. Int. 143, 105887 (2020).
3. Sarigiannis, D. et al. Advancing translational exposomics: bridging genome, exposome and personalized medicine. Hum. Genomics 19, 48 (2025).
4. Dop, C., Auvin, S., Mondot, S., Lepage, P. & Ilhan, Z. E. Longitudinal exposure to antiseizure medications shape gut-derived microbiome, resistome, and metabolome landscape. ISME Commun. 4, ycae123 (2024).
5. Healy, D. R. et al. Longitudinal associations of an exposome score with serum metabolites from childhood to adolescence. Commun. Biol. 7, 890 (2024).
6. Walker, D. I. et al. The Metabolome: a Key Measure for Exposome Research in Epidemiology. Curr. Epidemiol. Rep. 6, 93–103 (2019).
7. Sevelsted, A. et al. Effect of perfluoroalkyl exposure in pregnancy and infancy on intrauterine and childhood growth and anthropometry. Sub study from COPSAC2010 birth cohort. eBioMedicine 83, (2022).
8. Dunder, L. et al. Plasma levels of per- and polyfluoroalkyl substances (PFAS) and cardiovascular disease - Results from two independent population-based cohorts and a meta-analysis. Environ. Int. 181, 108250 (2023).
9. Stein, T. P., Schluter, M. D., Steer, R. A., Guo, L. & Ming, X. Bisphenol A Exposure in Children With Autism Spectrum Disorders. Autism Res. Off. J. Int. Soc. Autism Res. 8, 272–283 (2015).
10. Hyötyläinen, T. et al. In utero exposures to perfluoroalkyl substances and the human fetal liver metabolome in Scotland: a cross-sectional study. Lancet Planet. Health 8, e5–e17 (2024).
11. Hanson, M. A. & Gluckman, P. D. Early Developmental Conditioning of Later Health and Disease: Physiology or Pathophysiology? Physiol. Rev. 94, 1027–1076 (2014).
12. Miller, G. W. Integrating exposomics into biomedicine.
13. Vineis, P. et al. The exposome in practice: Design of the EXPOsOMICS project. Int. J. Hyg. Environ. Health 220, 142–151 (2017).
14. Jeong, A. et al. Perturbation of metabolic pathways mediates the association of air pollutants with asthma and cardiovascular diseases. Environ. Int. 119, 334–345 (2018).
15. Fiorito, G. et al. Oxidative stress and inflammation mediate the effect of air pollution on cardio- and cerebrovascular disease: A prospective study in nonsmokers. Environ. Mol. Mutagen. 59, 234–246 (2018).
16. Nassan, F. L. et al. Metabolomic signatures of the long-term exposure to air pollution and temperature. Environ. Health 20, 3 (2021).
17. Faquih, T. O. et al. Robust Metabolomic Age Prediction Based on a Wide Selection of Metabolites. J. Gerontol. A. Biol. Sci. Med. Sci. 80, glae280 (2025).
18. World Cancer Report: Cancer Research for Cancer Prevention. (International Agency for Research on Cancer, Lyon, 2020).
19. European Food Safety. Conclusion on the peer review of the pesticide risk assessment of the active substance diquat. EFSA J. 13, 4308 (2015).
20. Environmental Protection Agency. EPA’s Preliminary Supplemental Consideration of Certain Issues in Support of its Interim Registration Review Decision for Paraquat.
21. Lawlor, D. A., Tilling, K. & Davey Smith, G. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 45, 1866–1886 (2016).
22. Wan, M. et al. Exposomics: a review of methodologies, applications, and future directions in molecular medicine. EMBO Mol. Med. 17, 599–608 (2025).
23. He, C. et al. Effect of Diquat on gut health: molecular mechanisms, toxic effects, and protective strategies. Front. Pharmacol. 16, 1562182 (2025).
24. Lai, K. et al. Sensing of mitochondrial DNA by ZBP1 promotes RIPK3-mediated necroptosis and ferroptosis in response to diquat poisoning. Cell Death Differ. 31, 635–650 (2024).
25. Fu, Q., Tan, Z., Shi, L. & Xun, W. Resveratrol Attenuates Diquat-Induced Oxidative Stress by Regulating Gut Microbiota and Metabolome Characteristics in Piglets. Front. Microbiol. 12, 695155 (2021).
26. Magalhães, N., Carvalho, F. & Dinis-Oliveira, R. Human and experimental toxicology of diquat poisoning: Toxicokinetics, mechanisms of toxicity, clinical features, and treatment. Hum. Exp. Toxicol. 37, 1131–1160 (2018).
27. Sarigiannis, D. A. & Karakitsios, S. P. Addressing complexity of health impact assessment in industrially contaminated sites via the exposome paradigm. Epidemiol. Prev. 42, 37–48 (2018).

Download the Guide as a PDF
Explore the complexity and associated challenges of studying the exposome by downloading our complete Guide to the Exposome, which provides a comprehensive examination of the state of exposome research and how metabolomics can help generate meaningful insights in this field.
GET STARTED
Talk with an expert
Request a quote, get detailed information on sample types, or learn how metabolomics can accelerate your research. Find our contact details are here.
Find us on: