Advance your Preclinical and Clinical Research with Metabolomics
Focus your research
Advance Multi-omics Insights with Metabolomics

Solve for Biopharmaceutical Workflows
A recent paper in Current Opinion in Toxicology, titled “Confidence in Metabolite Identification Dictates the Applicability of Metabolomics to Regulatory Toxicology”, builds on this idea by suggesting for the purposes of toxicology studies that, “…such a multi-omics ‘triangulation’ of both upstream gene expression and downstream function of metabolites and lipids would not only help to substantiate the identities of the molecular entities but would provide greater confidence in the importance of a particular biochemical pathway in response to a chemical.”1 Metabolon’s unrivalled global library of metabolites and biopharma panels could help you more quickly and confidently draw conclusions from studies.
Some common questions metabolomics can answer include:
See how Metabolon can advance your path to preclinical and clinical insights
Enable Insight Across Drug Development Stages
Empower Effective Decision-making
Move Science Forward
Metabolon offers metabolomics services across the research continuum, from discovery to biomarker quantification and diagnostic test development. Our team of experts works with you to develop a tailored study design to meet your research needs across all areas of life sciences. Metabolon has pioneered Precision Metabolomics, the industry’s most comprehensive global metabolomics and lipidomics technology. We have built upon this technology and expertise to also offer the Complex Lipids Targeted Panel and pre-developed and custom-targeted panels for research and clinical trials.
Improve the Probability of Research Success
Many laboratories have metabolite profiling or analytical chemistry capabilities, but none have the extensive library of metabolite standards or informatics systems that power Metabolon’s platform. Metabolon has been granted over 200 patents covering its technology, methods and a growing portfolio of biomarkers. Our unmatched expertise and proprietary metabolomics systems ensure that our clients receive the most accurate, comprehensive picture of the metabolome.

Strategize Biomarker Development
Assure Data Integrity
Metabolon delivers state-of-the-art quality assurance and quality control at every stage, a rigorous data governance program, and adherence to valued U.S. federal and International standards and regulations.
Our robust Quality Management System (QMS) goes beyond internal quality assurance and quality control to include external certifications and adherence to those standards. Every procedure we perform, including sample management, data analysis, instrument maintenance, reagent qualification, and method validation is governed by an ISO 9001:2015 certified quality management system.
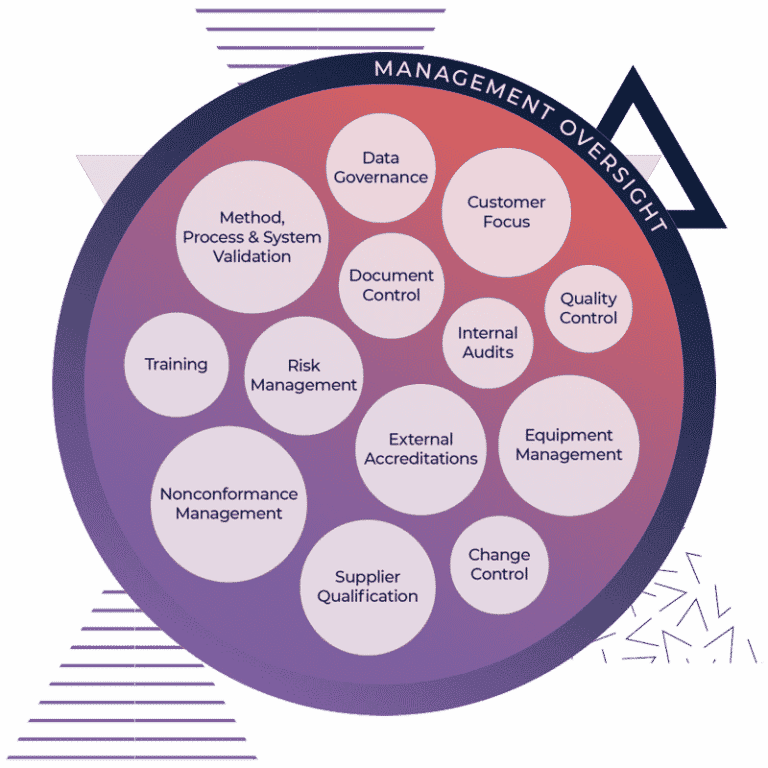

Apply Rigorous Data Governance
All the experimental and instrument data generated are automatically monitored by our quality-control software and then manually inspected and verified by our experienced lab personnel.
Our laboratory is Certified by Clinical Laboratory Improvement Amendments (CLIA) and accredited by College of American Pathologists (CAP) standards to perform diagnostic testing on human samples. We apply a rigorous Data Governance Program founded on industry-expected principles of data integrity, data privacy, data security, and adherence to applicable data privacy laws.
WILLETTE ET AL. 2021
How Metabolon Helps Improve Trial Design and Supports Trial Success
Merck Research Laboratories and Schering-Plough Research Institute—Markers of acute renal toxicity enable screening less toxic chemotypes
While in early development of a molecule directed at a novel target for lowering cholesterol, Dr Kevin B. Alton, Ph.D., and his team at Merck Research Laboratories worked with Dr Walter Korfmacher, Ph.D., Distinguished Fellow, and Dr Ganfeng Wang, Ph.D., Senior Scientist, at Schering-Plough Research Institute used metabolomics screening revealed markers associated with acute renal failure in mice. Conventional markers did not clearly signify the issue or explain it. The metabolomic biomarkers provided confidence that the effects were clearly off-target, and these markers were used to screen other chemotypes to show that the toxicity was isolated to a particular chemotype. This insight provided a potential way forward for the program. This example highlights the peril of relying solely on established markers as they were ineffective in reflecting and explaining the toxicity. In contrast, metabolomic fingerprinting provided clarity and a way forward.3,4
Gilead Sciences—Simple blood biomarkers for assessing response add to the development toolbox in NASH
Endpoints for nonalcoholic steatohepatitis (NASH) trials have many disadvantages including reliability. Simple blood biomarkers are highly desired. The need is amplified since there are so many novel targets in the NASH pipeline—quickly knowing if the program is heading in the right direction (ie, hitting the target, signs of efficacy) is critical. Leveraging metabolomics, Dr. Rohit Loomba, MD, MHSc, Director of NAFLD Research Center at UC San Diego, and his team collaborated with Gilead Sciences and others to bolster the confidence that they were on the right track in their successful phase II trial of GS-0976 by discovering several metabolite markers of efficacy. These biomarkers can accompany future development for reading out the pharmacodynamic activity or response of GS-0976.5
Interested in Further Studies?
Why Metabolon?
Once you see the full value of metabolomics, the only remaining question is who does it best? While many laboratories have metabolite profiling or analytical chemistry capabilities, comprehensive metabolomics technologies are extremely rare. Accurate, unbiased metabolite identification across the entire metabolome introduces signal-to-noise challenges that very few labs are equipped to handle. Also, translating massive quantities of data into actionable information is slow, if not impossible, for most because proper interpretation takes two things that are in short supply: experience and a comprehensive database.
Only Metabolon has all four core metabolomics capabilities
Coverage
Ability to interrogate thousands of metabolites across diverse biochemical space, revealing new insights and opportunities
Comparability
Ability to integrate the data from different studies into the same dataset, in different geographies, among different patients over time
Competency
Ability to inform on proper study design, generate high‐quality data, derive biological insights, and make actionable recommendations
Capacity
Ability to process hundreds of thousands of samples quickly and cost‐efficiently to service rapidly growing demand
Partner with Metabolon to access:
A library of 5,400+ known metabolites, 2,000 in human plasma, all referenced in the context of biochemical pathways
- That’s 5x the metabolites of the closest competitor
Unparalleled depth and breadth of experience analyzing and interpreting metabolomic data to find meaningful results
- 10,000+ projects with hundreds of clients
- 2,000+ publications covering 500 diseases, including numerous peer-reviewed journals such as Cell, Nature and Science
- Nearly 40 PhDs in data science, molecular biology, and biochemistry
Using our robust platform and visualization tools, our experts are uniquely able to tell you more about your molecule and develop assay panels to help you zero in on the results you need.
Related BioPharma Metabolomics Resources
Contact Us
Talk with an expert
Request a quote for our services, get more information on sample types and handling procedures, request a letter of support, or submit a question about how metabolomics can advance your research.
Corporate Headquarters
617 Davis Drive, Suite 100
Morrisville, NC 27560
Mailing Address:
P.O. Box 110407
Research Triangle Park, NC 27709
References
1. Malinowska JM, Viant MR. Confidence in metabolite identification dictates the applicability of metabolomics to regulatory toxicology. Current Opinion in Toxicology. 2019/08/01/ 2019;16:32-38. doi:10.1016/j.cotox.2019.03.006
2. Frédérich M, Pirotte B, Fillet M, de Tullio P. Metabolomics as a Challenging Approach for Medicinal Chemistry and Personalized Medicine. Journal of medicinal chemistry. 2016;59 19:8649-8666.
3. Zgoda-Pols JR, Chowdhury S, Wirth M, Milburn MV, Alexander DC, Alton KB. Metabolomics analysis reveals elevation of 3-indoxyl sulfate in plasma and brain during chemically-induced acute kidney injury in mice: investigation of nicotinic acid receptor agonists. Toxicol Appl Pharmacol. Aug 15 2011;255(1):48-56. doi:10.1016/j.taap.2011.05.015
4. Wang G, Korfmacher WA. Development of a biomarker assay for 3-indoxyl sulfate in mouse plasma and brain by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. Jul 2009;23(13):2061-9. doi:10.1002/rcm.4111
5. Loomba R, Kayali Z, Noureddin M, et al. GS-0976 Reduces Hepatic Steatosis and Fibrosis Markers in Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. Nov 2018;155(5):1463-1473.e6. doi:10.1053/j.gastro.2018.07.027

