Normal kidney function is essential for maintaining physiological homeostasis. The kidneys possess a diverse range of functions, including excretion, hormone production, blood pressure regulation, and pH balance.1 Unfortunately, chronic kidney disease (CKD) affects approximately 8 to 10% of individuals in Western countries, and diseases such as diabetes and obesity can exacerbate CKD pathology.
Combining the complexity of kidney physiology with the high comorbidity load, understanding CKD mechanisms, diagnosis, and treatment poses a significant challenge. CKD treatments are often invasive and expensive, and there are no known cures. In addition to CKD, other serious kidney diseases include diabetic nephropathy (the leading cause of CKD), acute kidney injury (AKI; characterized by a rapid loss of renal function), kidney cancer, and polycystic kidney diseases.2
As one of the biggest challenges in global health, there is an urgent need for accurate and sensitive tools to improve mechanistic understanding of kidney diseases, detect early-stage kidney disease biomarkers, and identify targetable molecules for treatment. Since current methods utilize a limited set of serum and urine biomarkers that lack sensitivity and specificity, metabolomics has emerged as an important tool for providing deeper insight into kidney function and kidney diseases.3
Challenges in Kidney Research
Existing technologies for kidney research and clinical decisions in CKD utilize a small set of serum and urine biomarkers such as serum creatinine and blood urea nitrogen, in addition to kidney histopathology and immunohistochemistry. Although serum creatinine is a cost-effective and commonly used biomarker, it has several limitations. For instance, it may not show changes until significant renal damage has occurred, it can overestimate renal function, it does not provide insights into underlying mechanisms or causes, and its levels can vary based on age, sex, body fluid status, muscle mass, and body weight.4
The narrow dynamic range of serum creatinine can significantly skew estimated glomerular filtration rate (eGFR; how well the glomerulus of the kidney is filtering blood), the current gold standard for measuring kidney function. Therefore, researchers and clinicians require tools that can provide a more accurate and sensitive assessment of kidney disease and that can detect kidney disease in early stages and monitor disease progression. Metabolomics provides a solution by enabling the generation of unbiased panels of novel biomarkers, allowing the quantification of alterations at a systems biology level.
Insights Gained from Metabolomics
In the past decade, the application of metabolomics has provided substantial insight into understanding the mechanisms underlying kidney disease as well as comprehensive collections of promising biomarkers that can be used for diagnosis, disease monitoring, and treatment. Numerous studies have shed light on metabolite pathways that are involved in kidney function, discovered novel clinical biomarkers for CKD, and enhanced clinical diagnosis of CKD.
Understanding the dysbiosis of the gut microbiome and related metabolites in CKD (Figure 1, adopted from Lohia et al., 2022), one report examined the association between alterations in CKD metabolites and gut microbiome alterations.6 Using a rat model of CKD, these researchers found a significant correlation between a decline in gut microbiome diversity and significant alterations in 291 serum metabolites, including lipids, amino acids, bile acids, and polyamines. Notably, CKD rats exhibited decreased levels of tyrosine and tryptophan that were also associated with changes in specific microbes.
In another study utilizing rat models of CKD, researchers found significant associations between CKD-induced kidney damage (measured through blood biochemistry and histopathology) and alterations in related metabolites.7 Altered metabolites associated with kidney injury included uremic toxins such as indoxyl sulfate and p-cresyl sulfate and nucleotide metabolites (e.g., xanthene). Interestingly, this report also uncovered a causal link between indoxyl sulfate and p-cresyl sulfate in driving increased kidney fibrosis through elevated expression of transforming growth factor-β1 (TGF- β1).
In investigations involving humans with mild, moderate, and severe tubulointerstitial lesions (i.e., diseases involving tubules and/or interstitium of the kidney), researchers employed metabolomics to generate panels associated with different stages of kidney damage.8 Interestingly, their findings revealed that the onset of tubulointerstitial lesions was associated with decreased levels of citrate, hippurate, glycine, and creatinine, while later deterioration was associated with complete depletion of citrate and hippurate, along with an increase in lactate, acetate, and trimethylamine-N-oxide. These results suggest that metabolomics has the potential to stratify biomarkers according to different stages of kidney disease, enabling early detection and diagnosis.
Other metabolomic studies focusing on CKD patients have successfully identified relevant metabolites based on disease stage. Applying metabolomics to human CKD serum from stage 4 CKD patients, one report demonstrated that metabolic alterations in glycolysis, amino acids, and organic osmolytes play an important role in CKD progression. Fluctuations in specific metabolite concentrations (e.g., glucose products, lactate, valine, glutamate, taurine) were found to be highly accurate and sensitive indicators of disease stage.9
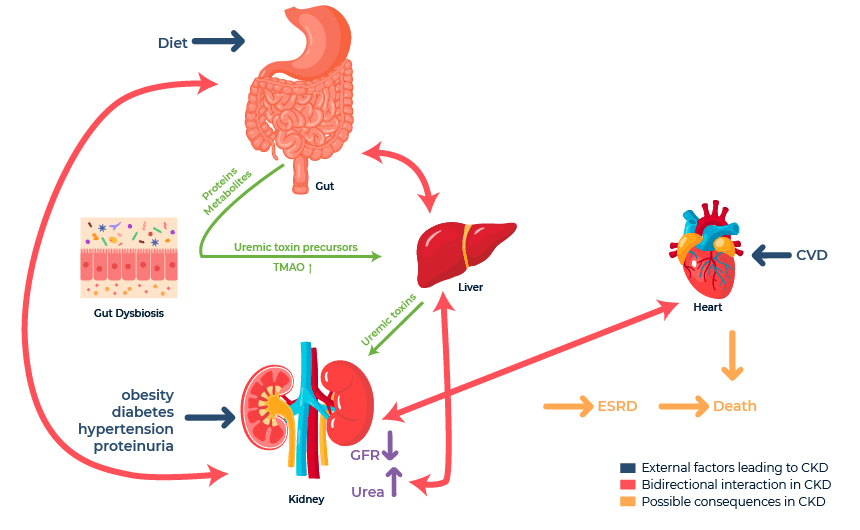
Figure 1. Interactions between gut, kidney, and metabolites.
Metabolon’s Kidney Discovery Panel
Metabolon recognizes the specific needs of researchers and clinicians dedicated to studying and treating kidney disease pathology. By leveraging metabolomics, Metabolon enables the generation of novel biomarkers offering crucial insights into kidney function and the biological processes influencing it. These insights present promising opportunities for enhancing kidney disease diagnosis, monitoring, and identifying therapeutic targets for early-stage interventions.
Metabolon’s Kidney Discovery Panel is uniquely positioned to quantify changes in 82 kidney-related metabolites across various biological pathways, including amino acids and derivatives (e.g., creatinine, tryptophan), carbohydrate metabolites (e.g. erythritol), lipids (e.g. 1-palmitoyl-2-linoleoyl-GPC), nucleotides (e.g., xanthine), and protein catabolism products (e.g., N6-acetyllysine). Contact us to learn more.

Kidney Function Discovery Panel
The Kidney Function Discovery Panel analyzes 84 metabolites associated with the biochemical processes that affect kidney function to help researchers differentiate the diverse spectrum of kidney disease.




