Most chemical compounds, including small molecules, generally have several different name attributes; their use depends on the context, preference, awareness and knowledge among others. Thus each of the chemicals can have common or simple names for familiarity, but can also be assigned other names based on scientific IUPAC nomenclature and other naming conventions that can result in inconsistensies of reporting, confusion, and potential challenges in understanding the importance of a specific chemical’s presence and impact on model systems across studies. Several institutions, such as Pubchem, the Human Metabolome Database (HMDB), Metabolomics Workbench, and the European Molecular Biology Laboratory (EMBL) have established collaborations in an attempt to harmonize the chemical names on a common database link with some limitations (e.g., inclusion of structural diversity related to stereocenters and isomers of a chemical compound). For example, leucine can be found in the D-form, the L-form, or as a generic form with no chirality specified depending on which specific database link individual users are using. This issue is particularly confounding in the area of lipid nomenclature.
Complex lipids have a lipid class that is dictated by the specific head group and one or more fatty acid tails. For example, the structure below is representation of a glycerophosphocholine lipid as the head-group is composed of a phosphocholine attached to a glycerol backbone. Other examples of lipid classes include phosphoinositols, diacylglycerols, sphingomyelins, and others. Each lipid also contains a number of fatty acids attached to the head group, one through four being the most common number. In the example below, a phosphocholine lipid has two fatty acids attached to the glycerol backbone. Different lipid classes have different numbers of fatty acids that can be attached, as well as different locations on the glycerol backbone where the fatty acids can attach. This combination of lipid class diversity, fatty acid diversity, and structural attachment diversity leads to a great deal of confusion when it comes to assigning a name to a lipid that has been detected in a traditional untargeted metabolomics analysis.
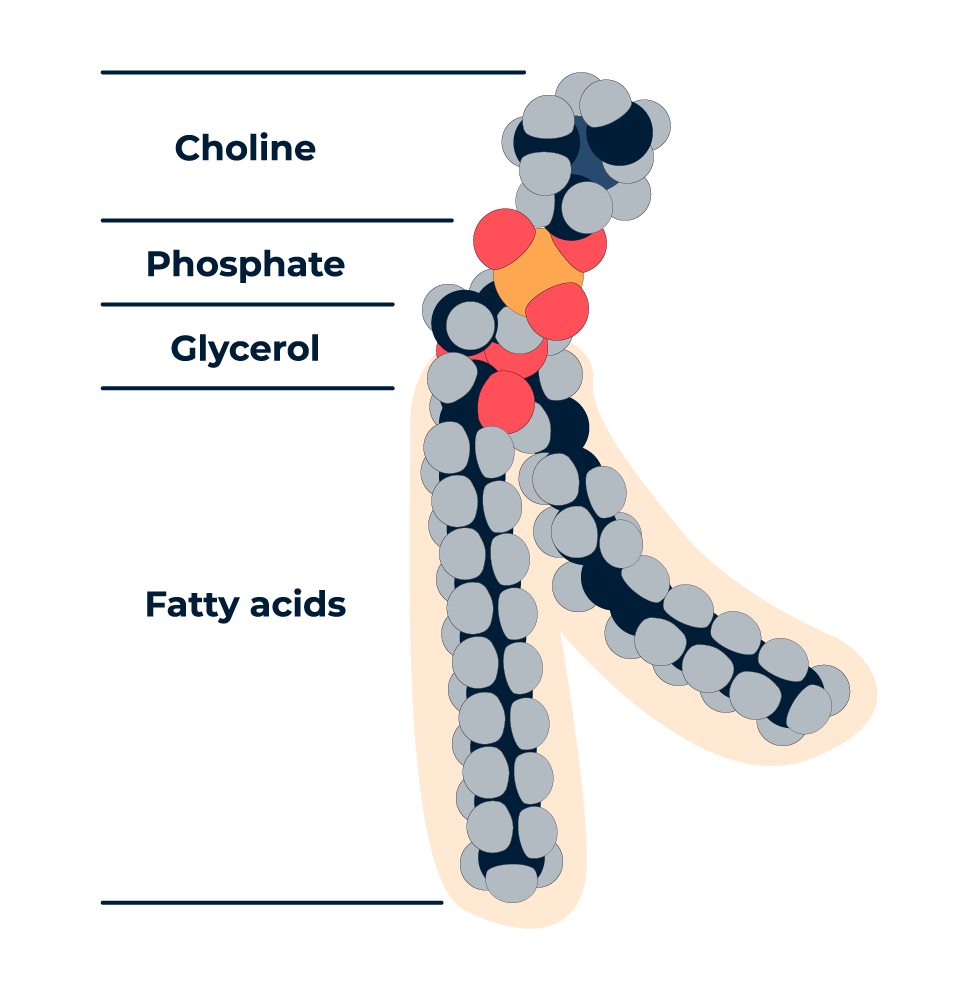
In addition to the diversity of complex lipid structures, the variety of analytical methods used in the field means that users have different capabilities to resolve these class, fatty acid, and structural differences. While some approaches can only resolve a structure to a basic level (e.g., phosphocholine with 34 carbons and zero double bonds), others can resolve to a more specific level (e.g., phosphocholine containing a palmitate and a stearate fatty acid). As a result, the names used to report the detection of lipids have become incredibly diverse, with little consistency across groups. A publication sponsored by LipidMaps in 2020 provides recommended guidelines and a framework for naming lipids.1
Over the coming months Metabolon will be updating our client deliverables to be consistent with new recommended guidelines1 and to aid in translatability of our data. Information regarding the class, fatty acid, and positional information will be provided in new columns included in Metabolon’s client deliverables. Given the number of lipids potentially affected, we will be releasing these updates in steps. These new columns will only have content for relevant lipid species and will otherwise be blank for non-lipids.
In Table 1 there are a few examples of the new columns displayed in the client deliverables. The chemID and chemical name of the entity will remain the same for historical tracking, but three new columns will be present, which are aligned with the LipidMaps publication.
- “Lipid Species Level” column—This column provides the shorthand lipid class designation (PC, PE, PI, etc.) followed by combined total number of carbons and double bonds in the fatty acid substituents.
- For example, PC 30:0 for a phosphocholine with a combined total of 30 carbons and zero double bonds in the bound fatty acids.
- This is a common shorthand used for lipids but does not provide details about the identity of the specific fatty acid species.
- “Lipid Molecular Species Level” column—This column provides the specific breakdown of which specific fatty acid species are present on the lipid, if known.
- For example, PC 30:0 is further known to be PC 14:0_16:0.
- “Lipid Sn Position Level”—The last piece of the structure is the location of the attachment of the fatty acid (sn-1, sn-2, etc). This final new column spells out the specific location of the fatty acids on the lipid backbone with the “/” separating the sn-1, sn-2, and sn-3 locations in that order.
- For example, 1-meadoylglycerol (20:3n9) is known to have the 20:3n9 fatty acid in the sn-1 position and so is designated as MG 20:3(5,8,11)/0:0/0:0.
- Note that the numbers within the parentheses indicate the location of the double bonds on the fatty acid backbone.
- In general, part of the new nomenclature is the use of an “_” rather than a “/” when the position of the fatty acid bonds on the headgroup are not known.
- For example, 1-meadoylglycerol (20:3n9) is known to have the 20:3n9 fatty acid in the sn-1 position and so is designated as MG 20:3(5,8,11)/0:0/0:0.
Table 1. Example of new columns
| Chemical ID (ChemID) | Chemical Name | Lipid Species Level | Lipid Molecular Species Level | Lipid Sn Position Level |
| 100000672 | 1-myristoyl-2-palmitoyl-GPC (14:0/16:0) | PC 30:0 | PC 14:0_16:0 | |
| 100019796 | 1-stearyl-2-oleoyl-GPE (O-18:0/18:1) | PE O-36:1 | PE O-18:0_18:1 | |
| 100001263 | 1-palmitoyl-GPC (16:0) | LPC 16:0 | LPC 16:0 | LPC 16:0/0:0 |
| 100001562 | 2-palmitoyl-GPC (16:0) | LPC 16:0 | LPC 16:0 | LPC 0:0/16:0 |
| 1525 | 1,2-dipalmitoleoyl-GPE (16:1/16:1) | PE 32:2 | PE 16:1_16:1 | PE 16:1/16:1 |
| 1528 | 1-palmitoyl-2-linoleoyl-GPI (16:0/18:2) | PI 34:2 | PI 16:0_18:2 | |
| 100020746 | 1-meadoylglycerol (20:3n9) | MG 20:3 | MG 20:3(5,8,11) | MG 20:3(5,8,11)/0:0/0:0 |
These new columns provide multiple benefits. The new columns allow data to be easily compared to historical publications regardless of the level of analytical rigor with which the historical data was reported. For example, if a publication contains only the “brutto” level identification, the Lipid Species Level column can be used for direct comparison. Additionally, they allow researchers to more easily examine the data for trends and insights hidden in the differing constituent fatty acids, the classes of lipids, and the individual species themselves.
These changes are a direct result of Metabolon’s commitment to continuous improvement and our drive to deliver our clients not just data about small molecules, but also the big insights these molecules enable.
References
1. Liebisch G, Fahy E, Aoki J, et al. Update on LIPID MAPS classification, nomenclature, and shorthand notation for MS-derived lipid structures. J Lipid Res. 2020;61(12):1539-1555. doi:10.1194/jlr.S120001025





