Validation
We deliver robust quality assurance and quality control at every stage with a rigorous data governance program and adherence to valued U.S. federal and international standards and regulations.
Our robust Quality Management System (QMS) goes beyond internal quality assurance and quality control to include external certifications and adherence to those standards.
Metabolon’s Quality Management System (QMS)
We believe that even a small error can have big consequences, so we operate using procedures established in our QMS to ensure that our complex deliverables are accurate and consistent.
Learn more about the elements of our commitment to the quality of your study:
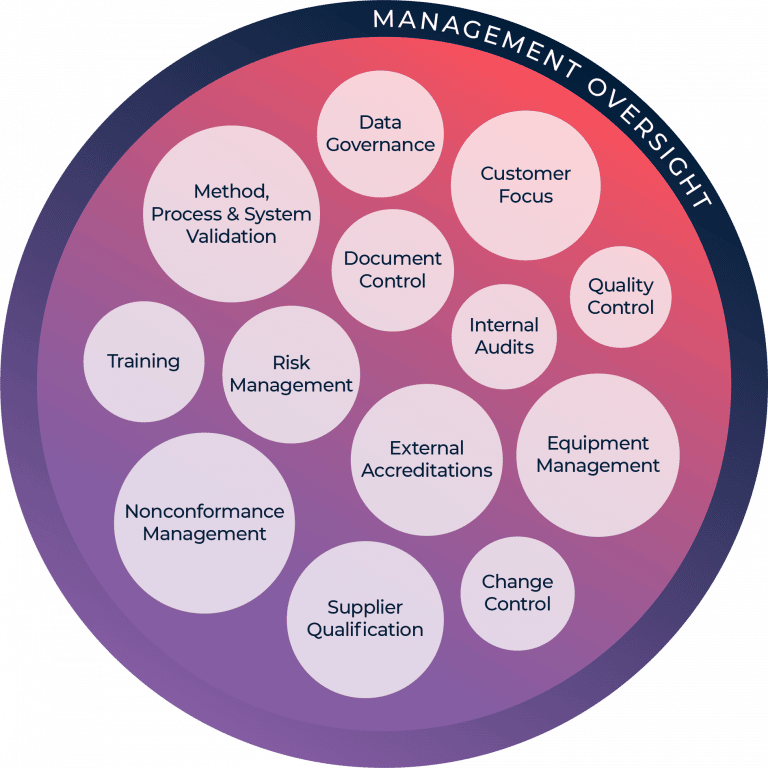

Metabolon’s Quality Policy
- A commitment to satisfy all applicable client requirements, regulations, and standards with ongoing oversight and continuous improvement of our quality processes.
- Ongoing investments in innovation to enable advancements in the field of metabolomics.
- Use of the most advanced technologies to deliver the highest quality data and biological interpretation in facilitating the understanding of health and disease.
Adherence to U.S. Federal and International Standards and Regulations
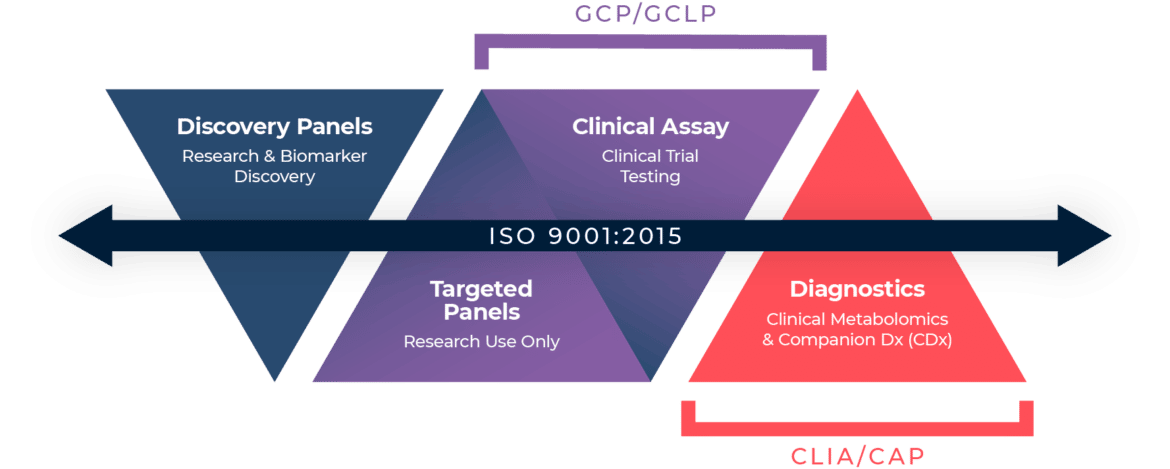
Metabolon has invested in developing and implementing internal processes and data governance polices that adhere to U.S. federal and international standards and regulations. Our rigor, established over 20 years, is especially critical for biopharma companies as they prepare data packages to support Investigational their New Drug (IND), Biologics License Application (BLA), and Marketing Authorisation Application (MAA) on the road to FDA or EMA regulatory approval.
We have the most comprehensive assortment of quality certifications among metabolomics providers to meet the diverse needs of our clients. Learn more about our certifications and quality standards:
ISO 9001:2015 – Quality Management Standard
ISO 9001:2015 is an international quality standard focused on maintaining good business practices with an emphasis on continuous improvement and customer satisfaction.
Click here to view our certificate
CAP – College of American Pathologists
Authorized by Center for Medicare and Medicaid Services (CMS) to inspect clinical laboratories and decide on accreditation to CLIA.
Click here to view our certificate
CLIA – Clinical Laboratory Improvement Amendments
These regulations focus on laboratory testing performed on specimens from humans for the purpose of diagnosis, prevention, or treatment of disease, or the assessment of health.
Click here to view our certificate
GCP (ICH E6) / GCLP (WHO GCLP/08) – Good Clinical Practice / Good Clinical Laboratory Practice
GCP standards support the conduct of clinical trials for any IND, BLA or MAA application. The International Council for Harmonisation (ICH) guidelines are focused on ensuring all GCP standards are followed for clinical trials across the globe. GCLP guidelines focus specifically on analytical testing performed on laboratory samples for clinical trials, which are not the focus of the ICH standards.
Metabolon follows required guidelines to meet FDA guidance on method validation and the parallel guidance to meet EMA regulatory requirements in Europe. These guidance documents focus on quantitative bioanalytical methods used to measure drug or metabolite concentrations in biological specimens obtained from non-clinical toxicology studies or from any clinical trial phase. They outline specific validation experiments to perform to demonstrate the analytical method is performing consistently within pre-established limits.
Because Metabolon adheres to these standardized recommendations for GCP testing, our clients can have confidence in the quality of data we provide to support their regulatory submissions. Any of Metabolon’s GCP studies also provide the added layer of GCLP quality applicable to bioanalytical studies.
Quality Control Throughout Your Study
Metabolon incorporates quality control steps at every stage of testing to ensure the highest quality data. Several types of analytical controls are utilized in concert with your study samples to ensure adequate assay and instrument performance. The type of controls used depends on the method employed. Metabolon also incorporates various quality control review steps to detect errors at all stages of data analysis and reporting to ensure the rigor and reproducibility of your study.
“We need to be able to stand behind the data because they are what will allow us to make claims on labels and go to regulators to support that these therapies are working in defined ways.”
– Christopher Ford, Ph.D., Seres Therapeutics
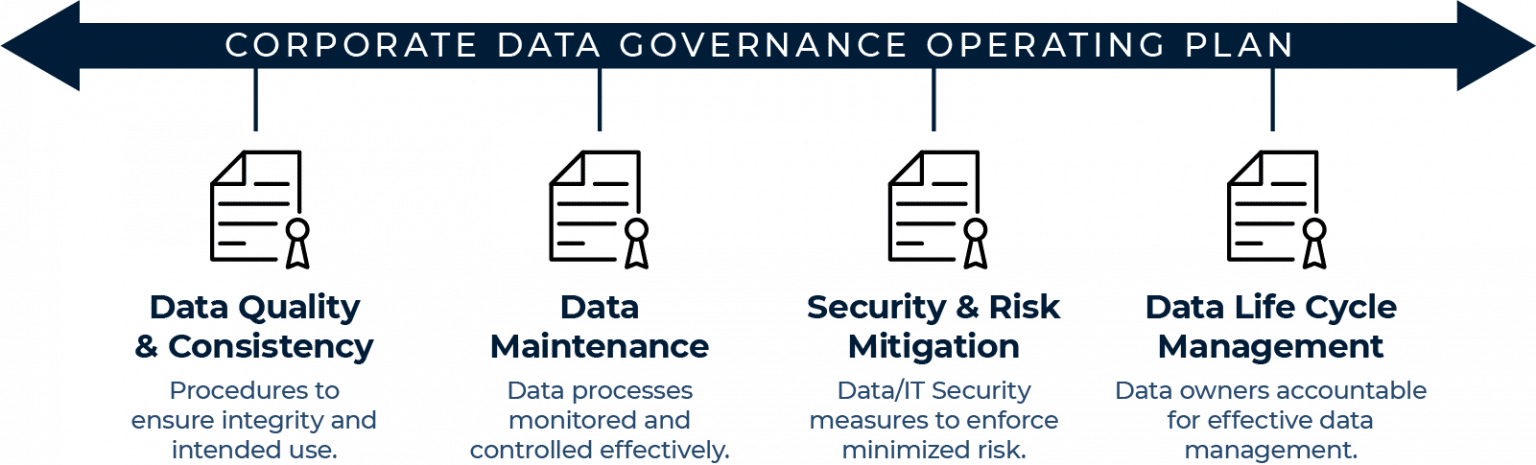
Rigorous Data Governance
Data Governance provides a plan to ensure data is available, usable, consistent, and secure.
Metabolon is committed to protecting all personal data we receive or collect to prevent misuse and unauthorized access and to complying with applicable data security and privacy laws and regulations. Metabolon’s Data Governance Program exists to establish risk-based safeguards that protect information while not impeding its appropriate use. You can find our full Privacy Policy here: https://www.metabolon.com/legal/
As part of our protection of data, Metabolon complies with regulatory mandates for handling personal data including the Health Insurance Portability Accountability Act (HIPAA), General Data Protection Regulation (GDPR), and California Consumer Privacy Act (CCPA). We have also implemented a comprehensive information security program based on the NIST Special Publication 800-53 Revision 4 that is fulfilled through various policies and procedures alongside physical and logical security controls.
High Quality Data and Client Support
With more than 20 years of experience in metabolite-based biomarker discovery and validation, Metabolon is equipped to assist companies to de-risk decision making and increase the success rate of their research efforts, including drug discovery and development.
Metabolon provides guidance to clients to ensure compliance with regulations and quality standards to support study integrity and high-quality data generation through all phases of the drug discovery and development process, from target selection to biomarker validation. We are here to ensure your study reaches its potential with the highest quality standards. This guidance is essential for biopharma supporting their need to accelerate pipelines and generate quality data for clinical trials founded in improving patient lives and outcomes. Our thousands of published studies, experience with hundreds of diseases and commitment to quality and data protection provide the rigor and confidence you need in your next study.
“Metabolon communicates at all stages, prompting me with the right questions to ensure we have a qualified process,” says Meaghan Sutton Bryant, Vendor Manager at Seres Therapeutics. “Even when we have encountered changes on our end, they showed they can be flexible while still keeping us on time. Communication is key with a vendor to take things off your plate that you do not need to be worrying about.”
Beyond the fact that our labs are ISO 9001:2015 certified, CLIA-certified and CAP-accredited for diagnostic testing on human specimens, our institutional knowledge will guide you through a successful metabolomics study from the start by ensuring your study is properly powered, properly controlled and properly aligned to your research & clinical objectives. The assay developed at Metabolon for your GCP study may also be available to support your patient testing in your post-approval commercialization phase.
Properly Powered
- Supported by the knowledge of our Ph.D. Discovery and Translational Science experts including our in-house biostatisticians, we begin with a study design to chart the best course for your study.
Properly Controlled
- The quality of your sample is everything and that’s why our team works with you to ensure that the guidelines for handling, storage and volumes are met.
Properly Aligned
- If you want the highest biological insights from your metabolomics study, you need the highest quality metabolite annotation. Our integrated bioinformatics solution makes it possible to efficiently and accurately mine the world’s most biologically relevant, validated metabolomics compound library for every study to ensure consistently reliable results every time. Learn more about Metabolon’s Tier 1 identification workflow.
Learn more about our process that helps to ensure you have the highest quality and reproducible data for your study.
Frequently Asked Questions
Does Metabolon offer bioanalytical testing that adheres to GCP standards?
Yes. Metabolon offers quantitative biomarker analyses of specific metabolites (also referred to as a targeted assay) that adhere to GCP and GCLP standards when this quality standard is required for clinical trials. All GCP/GCLP targeted assays are validated according to the FDA Bioanalytical Method Validation Guidance (2018) or the EMA Guidance for Bioanalytical Method Validation (2011). All other bioanalytical testing offered by Metabolon adheres to ISO 9001:2015 Quality Management Standards. ISO 9001:2015 outlines specific Quality Management System requirements with a strong focus on ensuring consistent and efficient processes, customer focus and satisfaction, and continual improvement.
Does Metabolon have the capability to develop quantitative biomarker assays that adhere to the FDA Bioanalytical Method Validation Guidance of 2018?
Yes. All GCP/GCLP targeted assays are developed and validated according to the rigorous requirements outlined in the FDA Bioanalytical Method Validation Guidance (2018). Metabolon also has the capability to validate GCP/GCLP targeted assays to the standards defined in the EMA Guideline on Bioanalytical Method Validation (2011).
What compliance and regulatory standards does Metabolon adhere to and which accreditations/certifications does Metabolon have?
Metabolon is ISO 9001:2015 certified for analytical and diagnostic testing of all biological samples. ISO 9001:2015 outlines specific Quality Management System requirements with a strong focus on ensuring consistent and efficient processes, customer focus and satisfaction, and continual improvement. Metabolon adheres to additional regulations or standards when performing specific types of testing:
Clinical Testing on Human Specimens: Metabolon adheres to the Clinical Laboratory Improvement Amendments (CLIA) regulations (42 CFR Part 493) when performing clinical testing on human specimens for the purpose of diagnosis, prevention, or treatment. Metabolon is also accredited by the College of American Pathology (CAP), which ensures that the laboratory does not only meet but also exceeds CLIA requirements. Metabolon possesses clinical laboratory licensures or permits from several states such as California, Maryland, Pennsylvania, and Rhode Island to perform clinical testing under CLIA. Metabolon holds a permit from New York State to perform specific tests under CLIA.
Our Certificates:
- CLIA – Clinical Laboratory Improvement Amendments
- CAP – College of American Pathologists
- ISO 9001:2015 – Quality Management Standard
If you need a certificate for a specific state, you may talk with your Metabolon client success team.
GCP/GCLP Testing: Metabolon has the capability to offer bioanalytical testing for targeted assays that adhere to GCP and GCLP quality standards.
Data Security and Data Privacy: Metabolon handles personal data in acordance with applicable data privacy laws and regulations. Metabolon has implemented a comprehensive information security program based on the NIST Special Publication 800-53 Revision 4 that combines policies and procedures with physical and logical security controls. Metabolon’s Information Security Officer and Data Privacy Officer, together with Metabolon’s Data Governance Steering Committee, are responsible for developing, maintaining and implementing the Data Governance Program in support of these program objectives.
Does Metabolon have a Data Governance and Data Privacy Program?
Yes. Metabolon’s Data Governance Program was established to support Metabolon’s commitment to protecting the personal data we receive or collect to prevent misuse and unauthorized access and to complying with applicable data security and privacy laws and regulations. The Data Governance Program establishes risk-based safeguards that protect information while not impeding its appropriate use. Metabolon’s Information Security Officer and Data Privacy Officer, together with Metabolon’s Data Governance Steering Committee, are responsible for developing, maintaining and implementing the Data Governance Program in support of these program objectives.
Metabolon handles personal data in acordance with applicable data privacy laws and regulations. Metabolon has implemented a comprehensive information security program based on the NIST Special Publication 800-53 Revision 4 that combines policies and procedures with physical and logical security controls.
Does Metabolon have the infrastructure in place to develop Diagnostics or Companion Diagnostics for a client or business partner?
Yes. Metabolon has the expertise and knowledge to partner with clients to develop Diagnostic (Dx) tests, including Companion Diagnostics (CDx). Metabolon collaborates with companies to develop and implement an appropriate regulatory strategy and ensures study integrity and high-quality data generation through all phases of the product development process. Metabolon employs a rigorous design control process to de-risk development and ensure that the Dx test meets all pre-established requirements.
Metabolon has the flexibility to develop and validate quantitative metabolomic diagnostic tests according to the stringent requirements of either the FDA Bioanalytical Method Validation Guidance or CLIA regulations and CAP standards, depending on the purpose of the Dx test. Following analytical validation of the diagnostic test, Metabolon has the capability to perform bioanalytical testing under GCP/GCLP or CLIA requirements to support clinical validation of the Dx or CDx.
Have More Questions?
See how Metabolon can advance your path to preclinical and clinical insights
Contact Us
Talk with an expert
Request a quote for our services, get more information on sample types and handling procedures, request a letter of support, or submit a question about how metabolomics can advance your research.
Corporate Headquarters
617 Davis Drive, Suite 100
Morrisville, NC 27560
Mailing Address:
P.O. Box 110407
Research Triangle Park, NC 27709

