Lanosterol
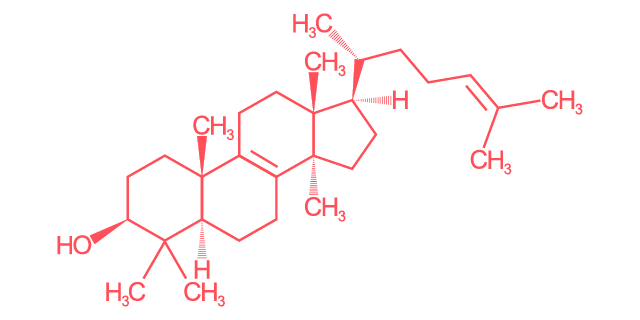
Linear Formula
C30H50O
Synonyms
n/a
Share this metabolite
Lanosterol is a tetracyclic triterpenoid and amphipathic molecule1. While dietary lanosterol can be obtained from meat and other food products, lanosterol formation also occurs endogenously through squalene synthase, and is particularly enriched in the lenses of the eyes. Notably, lanosterol serves as a vital precursor during cholesterol production, an upstream process to the formation of oxysterols, bile acids, and steroid hormones. This typically takes place in liver and intestinal cells, where metabolic end products and intermediaries are shuttled into circulation to various organs2. Collectively, lanosterol and its associated metabolites have widespread physiological functions.
Controlling lanosterol levels is crucial for maintaining cholesterol homeostasis. This tightly regulated process governs the integrity of cell membranes, as well as steroid hormone and bile acid levels. As a result, alterations in lanosterol and cholesterol have been implicated in numerous diseases including metabolic disorders, cardiovascular disease, and neurodegenerative diseases3.
Lanosterol and ophthalmology
Lanosterol has also gained considerable attention in ophthalmology due to its enrichment in the ocular lens and its role in maintaining the clarity of the lens 4. Maintenance of crystallin protein levels in lens fibers within the lens nuclei is critical for normal vision, and aggregation of these proteins is the main contributing factor for cataract development. Alterations in the LSS gene, which encodes lanosterol synthase, have been tied to protein aggregation and cataract formation in animal models and in humans. For example, rats with a mutation in LSS exhibit decreased cholesterol levels in the lens, resulting in cataract formation5. Furthermore, extensive work in humans has sought to characterize LSS mutations associated with congenital cataracts4.
Interestingly, lanosterol (but not cholesterol) treatment significantly decreases protein aggregation in vitro. Additionally, lanosterol treatment reduces lens opacity and cataract severity in dissected rabbit lenses in vitro and in vivo in dogs. Scientists suspect that lanosterol can restore vision by dissolving aggregated proteins and increasing lens clarity, but existing research is conflicting. For instance, an in vitro study found that lanosterol and other oxysterols fail to bind to crystalline chaperone proteins in rat and aged human lenses6, while in other studies, immersion of cataractous lens nuclei in lanosterol solution resulted in no significant changes in lens opacity after six days7.
Lanosterol and metabolic health
Given that lanosterol initiates cholesterol formation, it is unsurprising that this metabolite also plays a role in maintaining metabolic health. For example, elevated lanosterol levels have been found in obese children, along with an increased waist-to-hip ratio, body fat percentage, and BMI8. Similarly, elevated lanosterol levels have also been linked to impaired glucose metabolism in adolescents with type-2 diabetes9. Conversely, in a group of participants undergoing a two-year weight loss program, fluctuations in markers of cholesterol formation were accompanied by significant decreases in lanosterol levels during the active period of weight loss10. Unfortunately, these were followed by a robust rebound, leading to significantly increased lanosterol levels at two years compared to baseline.
Collectively, these findings suggest that an increase in lanosterol corresponds with an increase in cholesterol production. This triggers a cascade of biochemical imbalances, including elevated bile acids and steroids, which ultimately contribute to co-morbidities often observed in obesity and type-2 diabetes, such as inflammation and coronary heart disease11. Notably, administration of TNF-α, an inflammatory cytokine elevated in metabolic disorders, significantly alters both cholesterol formation and lanosterol levels.
Lanosterol and gastrointestinal health
Dysbiotic gut microbiota has emerged as a key contributor to metabolic disorders and overall health. A wealth of emerging research has linked gut microbiome diversity with fluctuations in metabolite levels, including those involved in cholesterol formation12.
For instance, knockout of Niemann-Pick C1-like 1 (NPC1L1), an essential cholesterol transporter, reduces stool output in mice, suggesting an important relationship between cholesterol and gastrointestinal health. Interestingly, microbiome diversity in NPC1L1 knockout mice is significantly altered compared to control mice. Furthermore, germ-free mice show decreased intestinal absorption and an increased excretion of cholesterol13.
Supporting these findings, a study using germ-free or antibiotic-treated zebrafish showed a significant interaction between LSS and the presence of intestinal bacteria14. These data suggest that the absence of a microbiome induces cholesterol loss leading to a compensatory increase in cholesterol precursors like lanosterol.
Lanosterol and neuroscience
Cholesterol also plays a critical role in maintaining the structural integrity of cell membranes, with particular importance in the brain, where it constitutes 23% of the body’s sterol content15. Recent research has highlighted the essential roles of lanosterol and other cholesterol intermediates in brain cholesterol metabolism. These functions carry implications for several neurodegenerative diseases.
Myelin, which forms around neuronal axons to facilitate neurotransmission, accounts for 70% of brain cholesterol16. When the brain lacks cholesterol, neurotransmission and synaptic plasticity fails17. Furthermore, lanosterol is significantly decreased in the hippocampus of the elderly compared to younger subjects18.
Other studies have shown that cholesterol formation is impaired in several neurodegenerative diseases. For instance, individuals affected by Huntington’s Disease exhibit significant reductions in lanosterol and other cholesterol precursors19. Moreover, the protective effects of lanosterol have been demonstrated in Parkinson’s disease, where lanosterol administration safeguards dopaminergic neurons by regulating mitochondrial function20.
Lanosterol and drug development
Lanosterol has garnered significant attention as a potential therapeutic approach for cataracts. Given that the current treatment primarily revolves around cataract surgery, the exploration of lanosterol as a non-surgical drug, often in the form of non-invasive eye drops, presents an appealing avenue for ophthalmologists seeking alternative treatment options.
Using this metabolite to treat cataracts should be approached cautiously, however. While one group investigating Lanomax, a lanosterol-based eye drop for animals, found that lanosterol concentrations remained constant for twelve hours and later used the drug to treat cataracts in a single elderly person with severe cataracts21, most work reveals inconsistent efficacy. Therefore, much more work is needed to fully evaluate the utility of using this metabolite to clear cataracts.
Lanosterol in research
As of August 2023, there are over 2500 citations for “lanosterol” and 168,000 citations for “cholesterol synthesis” in research publications (excluding books and documents) on Pubmed. The tremendous number of publications in the last few years has linked this metabolite to a broad range of physiological functions (many of which are discussed here) suggests that any research program seeking to better understand ocular, metabolic, gastrointestinal, and neurological health may benefit from quantitative analysis of lanosterol. Preclinical research may also benefit from lanosterol quantification for a comprehensive understanding of biomarkers, diagnosis, and disease monitoring.
References
- Wishart, DS, Guo, A, Oler, E, et al. HMDB 5.0: the Human Metabolome Database for 2022. Nucleic Acids Res 2022;(50):D622-D631.
- Dietschy, JM, Turley, SD, and Spady, DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res 1993;(34):1637-1659.
- Gliozzi, M, Musolino, V, Bosco, F, et al. Cholesterol homeostasis: Researching a dialogue between the brain and peripheral tissues. Pharmacol Res 2021;(163):105215.
- Zhao, L, Chen, XJ, Zhu, J, et al. Lanosterol reverses protein aggregation in cataracts. Nature 2015;(523):607-611.
- Mori, M, Li, G, Abe, I, et al. Lanosterol synthase mutations cause cholesterol deficiency-associated cataracts in the Shumiya cataract rat. J Clin Invest 2006;(116):395-404.
- Daszynski, DM, Santhoshkumar, P, Phadte, AS, et al. Failure of Oxysterols Such as Lanosterol to Restore Lens Clarity from Cataracts. Sci Rep 2019;(9):8459.
- Shanmugam, PM, Barigali, A, Kadaskar, J, et al. Effect of lanosterol on human cataract nucleus. Indian J Ophthalmol 2015;(63):888-890.
- Son, HH, Kim, SH, Moon, JY, et al. Serum sterol profiling reveals increased cholesterol biosynthesis in childhood obesity. J Steroid Biochem Mol Biol 2015;(149):138-145.
- Semova, I, Levenson, AE, Krawczyk, J, et al. Markers of cholesterol synthesis are elevated in adolescents and young adults with type 2 diabetes. Pediatr Diabetes 2020;(21):1126-1131.
- Leichtle, AB, Helmschrodt, C, Ceglarek, U, et al. Effects of a 2-y dietary weight-loss intervention on cholesterol metabolism in moderately obese men. Am J Clin Nutr 2011;(94):1189-1195.
- Duan, Y, Gong, K, Xu, S, et al. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal Transduct Target Ther 2022;(7):265.
- Jia, B, Zou, Y, Han, X, et al. Gut microbiome-mediated mechanisms for reducing cholesterol levels: implications for ameliorating cardiovascular disease. Trends Microbiol 2023;(31):76-91.
- Zhong, CY, Sun, WW, Ma, Y, et al. Microbiota prevents cholesterol loss from the body by regulating host gene expression in mice. Sci Rep 2015;(5):10512.
- Sheng, Y, Ren, H, Limbu, SM, et al. The Presence or Absence of Intestinal Microbiota Affects Lipid Deposition and Related Genes Expression in Zebrafish (Danio rerio). Front Microbiol 2018;(9):1124.
- Dietschy, JM, and Turley, SD. Cholesterol metabolism in the brain. Curr Opin Lipidol 2001;(12):105-112.
- Laatsch, RH, Kies, MW, Gordon, S, et al. The encephalomyelitic activity of myelin isolated by ultracentrifugation. J Exp Med 1962;(115):777-788.
- Abbiss, H, Maker, GL, and Trengove, RD. Metabolomics Approaches for the Diagnosis and Understanding of Kidney Diseases. Metabolites 2019;(9).
- Thelen, KM, Falkai, P, Bayer, TA, et al. Cholesterol synthesis rate in human hippocampus declines with aging. Neurosci Lett 2006;(403):15-19.
- Leoni, V, Mariotti, C, Nanetti, L, et al. Whole body cholesterol metabolism is impaired in Huntington’s disease. Neurosci Lett 2011;(494):245-249.
- Lim, L, Jackson-Lewis, V, Wong, LC, et al. Lanosterol induces mitochondrial uncoupling and protects dopaminergic neurons from cell death in a model for Parkinson’s disease. Cell Death Differ 2012;(19):416-427.
- L. M. Balashova, VAN, I. I. Kolesnichenko, V. V. Novoderyozhkin, & S. N. Udaltsov Lanomax as a Drug in Cataract Treatment: A Case Study. COMPLEX SYSTEMS BIOPHYSICS 2018;(63):655-661.

