Case Study
Using Metabolomics to Identify a Key Pathway in Lung Cancer Epithelial to Mesenchymal Transition (EMT) and Drug Resistance
Metabolon helps a leading provider of life-saving medicines developed with biotechnology to connect metabolic rewiring with EMT and acquired drug resistance in lung cancer.
Metabolon helped scientists employ a systems biology approach to gaining mechanistic insight behind EMT in lung cancer.
Metabolon helped scientists employ a systems biology approach to gaining mechanistic insight behind EMT in lung cancer.
The Challenge
Lung cancer is one of the most common cancers in the world. It grows quickly and metastasizes early, often to the liver, adrenal glands, bone, or brain. EMT—the transformation of epithelial cells into mesenchymal stem cells—facilitates cancer metastasis. It has also been implicated in acquired resistance to anti-cancer drugs, but the precise mechanisms behind this transformation have been a major challenge in cancer therapy. To address this challenge, Metabolon helped scientists employ a systems biology approach to gaining mechanistic insight behind EMT in lung cancer.1
The Metabolon Insight
The common mechanism by which cancer cells acquire drug resistance is through circumventing biochemical pathways, and metabolomics is the best technology to survey those pathways. Using metabolomics, we can identify new targets to limit cancer’s potential to develop drug resistance.
Combining global metabolomics data generated by Metabolon with transcriptomics data from 3 different lung cancer cell lines, the scientists identified evidence of a metabolic rewiring event causing glucose to divert to the TCA cycle. Glutamate accumulation in all 3 cell lines was due to this diversion of glucose. Additionally, PDK4, a gatekeeper of the TCA cycle, was consistently downregulated during EMT, and RNAi knockdown of PDK4 drove EMT and promoted erlotinib resistance in EGFR mutant lung cancer lines.
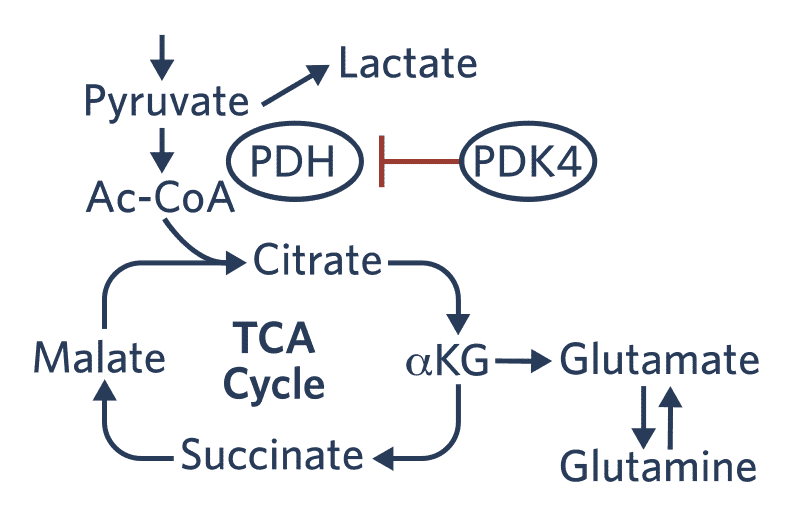
Figure 1. A combination of metabolomics and gene expression data revealed a metabolic switch during EMT that appeared to be driven by downregulation of PDK4.
Cancer Metab 2014. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited.
The scientists proposed that the metabolic switch to the TCA cycle provides an alternative method for cancer cell growth and survival during the stress of drug treatment, thereby facilitating acquired drug resistance during EMT. Assessing clinical samples from tumors, they found that PDK4 is downregulated in most tumor types, and PDK4 downregulation was a predictor of poor prognosis in lung cancer.
The Value of Discovery
The experimental results showed that downregulation of PDK4 in lung cancer is more substantial and prevalent than that of most other known tumor suppressors, leading the research team to propose PDK4 as a metabolic tumor suppressor. Their research revealed for the first time a unique metabolic reprogramming event key for EMT and acquired drug resistance. This finding represents a promising new area for therapeutics development, particularly because, based on the experimental results, downregulation of PDK4 expression appears well prior to metastasis. Targeting PDK4 early in lung cancer could play a critical role in metastasis prevention and improved survival outcomes.
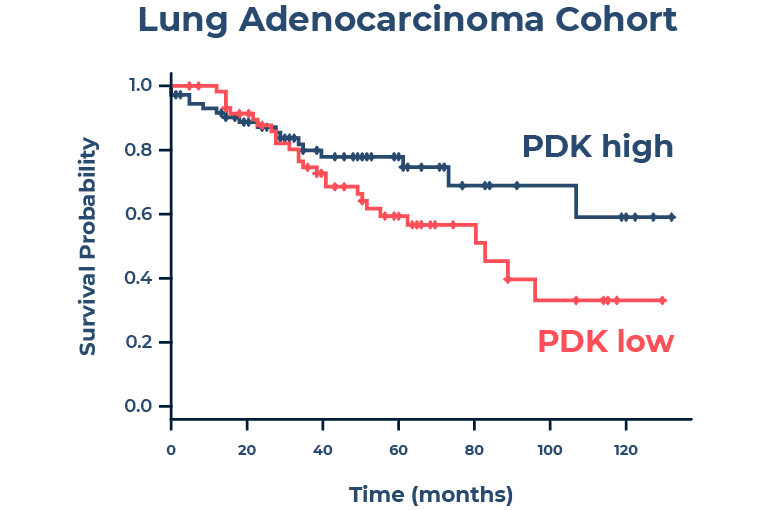
Figure 2. Downregulation of PDK4 (PDK low) was found to be a predictor of poor prognosis in lung cancer.
Cancer Metab 2014. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited.
References
1. Sun Y, Daemen A, Hatzivassiliou G, et al. Metabolic and transcriptional profiling reveals pyruvate dehydrogenase kinase 4 as a mediator of epithelial-mesenchymal transition and drug resistance in tumor cells. Cancer Metab. 2014;2(1):20. Published 2014 Nov 3. doi:10.1186/2049-3002-2-20