Complex Lipids Targeted Panel
Specificity of 1,100 Lipid Species
Accurate quantitation of 14 lipid classes
Complete coverage of fatty acid composition
Pathway Mapping and Discovery Tools for easy interpretation
About the Complex Lipids Targeted Panel
Complex lipids are a diverse class of metabolites that serve many functions in biology and play an important role in the development of metabolic diseases, cancer, inflammation, and central nervous system disorders.
Accurate measures of lipids are essential for biomarker discovery and for clarifying biological questions. But, because they have a diverse array of chemical structures and a high degree of isomeric overlap, lipids are exceptionally challenging to accurately identify and quantify. The obstacles inherent in lipid profiling make high-quality lipidomic platforms extremely rare.
The Complex Lipids Targeted Panel: Complete, Quantitative Lipidomic Analysis
Metabolon has overcome the challenges of lipid profiling to create the only lipidomic platform able to provide both quantitative compositional analysis and complete speciation data: the Complex Lipids Targeted Panel.
By combining the Sciex SelexION differential mobility spectrometry (DMS) with an extensive library of lipid masses and more than 50 custom-synthesized internal standards, the Complex Lipids Targeted Panel identifies up to 1,100 individual lipid species.
This unique platform provides single-point quantitation of 14 lipid classes, including principal phospholipid, sphingolipid, and neutral lipid classes. It also provides molecular species concentrations and the complete fatty acid composition of each lipid class, thereby offering unparalleled insight into the lipidome.
This exceptional data is then imported into our proprietary Surveyor Web Tools for intuitive data visualization and easier interpretation. The combination of data and tools represents a unique environment for understanding complex lipid metabolism—a true “next generation” lipidomic solution.
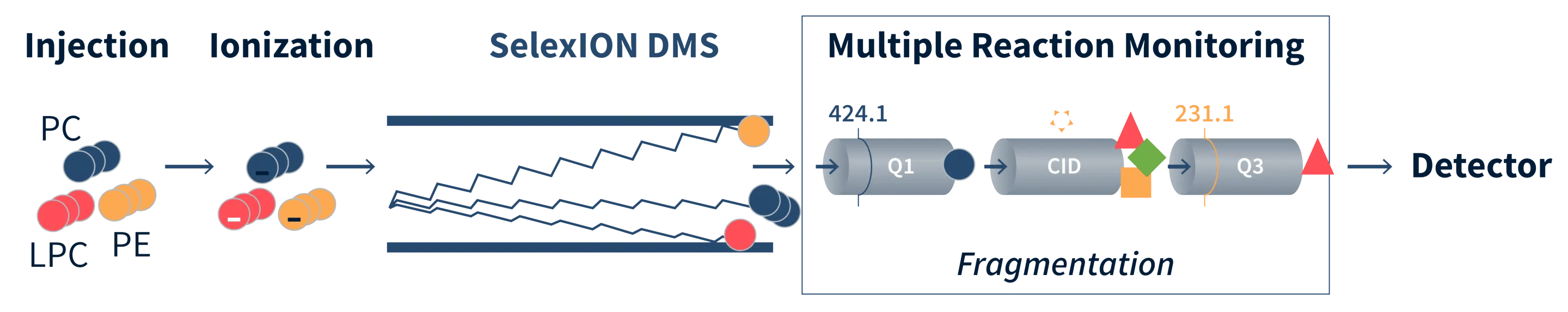
Delivering Absolute Quantification for Research and Biomarker Analysis
Our readily available or custom developed quantitative assays help you achieve your research and biomarker validation objectives with precise and fully validated methods. Our targeted assays and panels cover >1,000 metabolites and lipids across a wide range of biochemical classes, metabolic pathways, and physiological processes, and they can be customized to best fit any application.
Lipidomics Methods Eliminate Quantitative Bias
Lipids are extracted from samples in the presence of internal standards. The extracts are concentrated under nitrogen and reconstituted in dichloromethane:methanol (1:1) with 10 mM ammonium acetate.
The extracts are transferred to inserts and placed in vials for infusion-MS analysis, performed on a Shimadzu LC with nano PEEK tubing and the Sciex SelexION-5500 QTRAP. The samples are analyzed via both positive and negative mode electrospray. The 5500 QTRAP scan is performed in MRM mode with a total of more than 1,100 MRMs.
Individual lipid species are quantified by taking the intensity ratios of target compounds and their assigned internal standards, then multiplying by the concentration of internal standards added to the sample. Lipid class concentrations are calculated from the sum of all molecular species within a class, and fatty acid compositions are determined by calculating the proportion of each class comprised by individual fatty acids.
Full Coverage of Complex Lipid Metabolism
The Complex Lipids Targeted Panel provides the lipid concentration (eg, TAG), fatty acid composition, and molecular species (eg, PC(16:0/22:6)) of each of the 14 covered lipid classes.
| Class | Number of Species |
|---|---|
| Ceramide (CER) | 12 |
| Cholesteryl Esters (CE) | 26 |
| Diacylglycerols (DAG) | 58 |
| Dihydroceramide (DCER) | 12 |
| Hexosylceramide (HCER) | 12 |
| Lactosylceramide (LCER) | 12 |
| Lysophosphatidylcholine (LPC) | 26 |
| Lysophosphatidylethanolamine (LPE) | 26 |
| Monoacylglycerol (MAG) | 26 |
| Phosphatidylcholine (PC) | 140 |
| Phosphatidylethanolamine (PE) | 216 |
| Phosphatidylinositol (PI) | 28 |
| Sphingomyelin (SM) | 12 |
| Triacylglycerols (TAG) | 519 |
Our Custom Tools Transform Data into Knowledge
In addition to a quantitative results table, the Complex Lipids Targeted Panel also comes with access to Surveyor, a suite of custom-designed data visualization and analysis software tools. Two primary tools exist within Surveyor for interpreting the data—Pathway Mapping Tools and Discovery Tools.
Pathway Mapping Tools
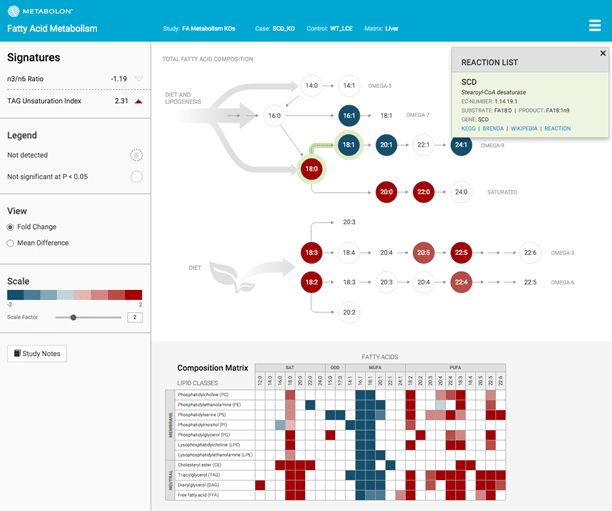
The Surveyor pathway maps allow clients to understand their lipidomic data in the context of lipid metabolic pathways.
Each pathway has been curated and annotated by Metabolon experts, and each element of the map is interactive, providing both data analysis (boxplots and difference testing) and additional information on each metabolite or activity.
Using Pathway Mapping tools, researchers can investigate the effect of their comparisons on the concentration and fatty acid composition of different pathways, including:
Complex Lipid Metabolism (phospholipids and neutral lipids)
Sphingolipid Metabolism (ceramides and sphingomyelin)
Fatty Acid Metabolism
Discovery Tools
Discovery tools allow clients to perform an unbiased evaluation of the performance of each lipid, and layer in biological information and structure when desired. The tools plot all of the measured lipids and allow clients to filter and sort results to aid in the identification of biomarkers.
The Biomarker Performance page provides a comprehensive view of the performance of each lipid marker in separating the treatment groups.
The Time Course/Multiple Comparisons page improves the analysis of time-course and multiple-treatment comparisons studies by allowing clients to simultaneously plot multiple comparisons between groups.
In both tools, each plot point is interactive, linking a selected lipid marker to additional information about their structure and biology, including related box plots.
Complex Lipids Targeted Panel Technical Whitepaper
Complex lipids are highly diverse, with over 40,000 unique structures documented in the Lipid Maps Structure Database. This technical white paper introduces the field of lipidomics and discusses the structure, nomenclature, and importance of complex lipids. It highlights the three main requirements for lipid analysis, including coverage, quantification, and specificity, and how Metabolon’s Complex Lipids Targeted Panel fulfills these requirements. Discover how Metabolon’s proprietary workflow is optimized for efficiency to provide high-quality results and learn about our robust data post-processing and interpretation. Partner with Metabolon to unleash the power of lipid analysis and advance your research.

Big Insights with Metabolon
Cited in over 3,000 publications, we help scientists and manufacturers gain greater insight into their studies through metabolomics. See how our approach can become a successful part of your workflow.
Related Metabolomics Resources
Contact Us
Talk with an expert
Request a quote for our services, get more information on sample types and handling procedures, request a letter of support, or submit a question about how metabolomics can advance your research.
Corporate Headquarters
617 Davis Drive, Suite 100
Morrisville, NC 27560
Mailing Address:
P.O. Box 110407
Research Triangle Park, NC 27709